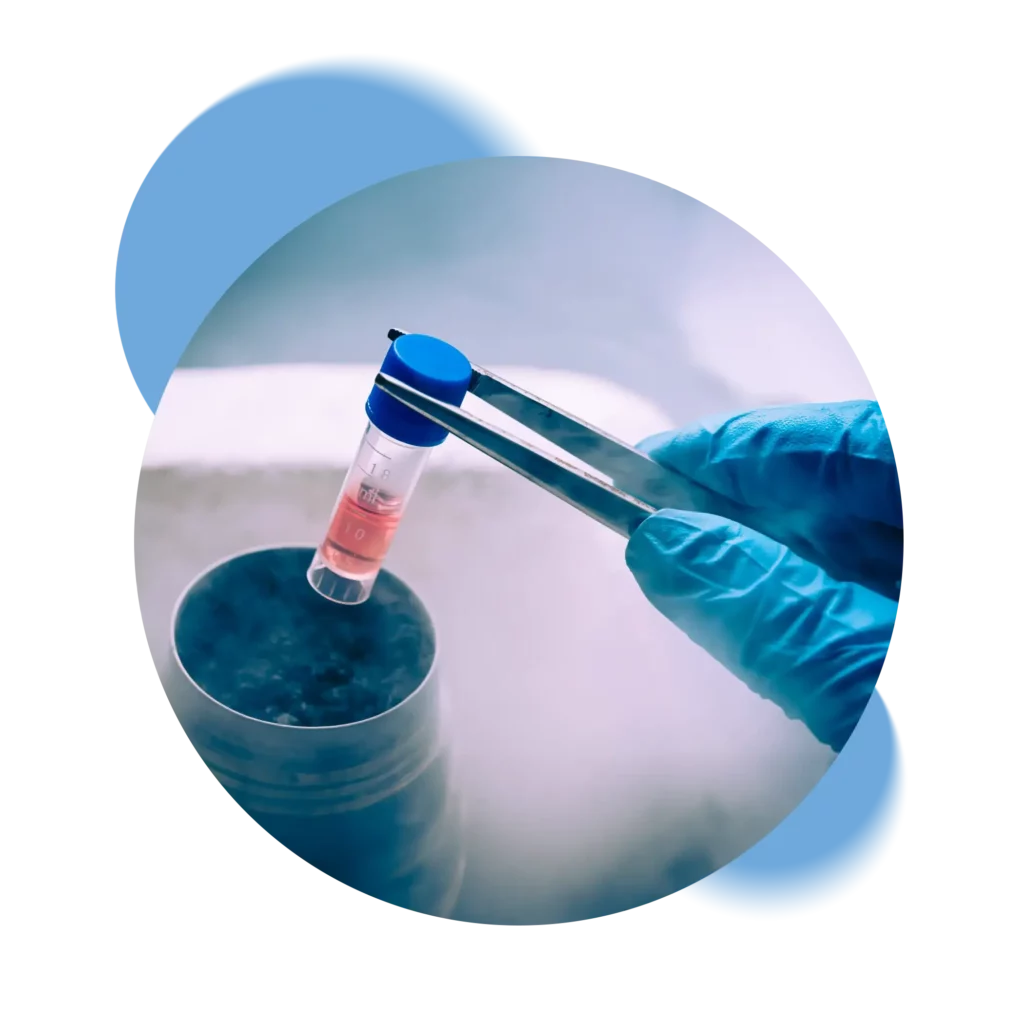
Your biological material is transferred directly from the delivery room to our laboratories, where it is processed and subsequently cryopreserved in Switzerland – unlike our competitors who transfer or process abroad.
Store cord blood for your family and make it available for those in need. A double value in one choice.
Switzerland’s only private Biobank that processes and stores samples exclusively in its own laboratory without going through intermediaries.
About Us
Swiss Stem Cells Biotech (SSCB), founded in 2005 in Lugano, Switzerland, is the only private Swiss biobank accredited by FACT-NetCord and certified GMP. Following strict Swiss regulations, SSCB handles the processing and cryopreservation of stem cells from cord blood, cord tissue, and adipose tissue. SSCB is the only biobank participating in the Swiss public-private hybrid banking pilot project.
Through this initiative, samples from patients who choose to participate in this biobanking model can be anonymously entered into the global registry of the World Marrow Donor Association (WMDA), making them potentially available anonymously for worldwide use. Parents can still decide whether they wish to donate the sample or not. No private sample will ever be shared with the public registry without prior explicit consent, and this model is only available at specific partner hospitals for birth.


Expertise
Storing the stem cells from umbilical cord and placental tissue represents an act of love for your baby. These cells have the ability to differentiate into various cell types, offering an unparalleled opportunity in the field of medicine.
SSCB Hybrid Model
The service represents an ideal solution that combines family safety with an act of solidarity towards other patients awaiting transplants, offering personal protection and a contribution to the community.
Since 2020, this project, approved by the Federal Office of Public Health (FOPH) and developed in collaboration with the Women’s Clinic of Inselspital in Bern, the Blood Transfusion Service of the Swiss Red Cross (SRC), and Swiss Stem Cell Biotech AG (SSCB), offers a hybrid storage service available in various clinics nationwide.

A vital resource for medicine, with applications for established therapies in more than 80 diseases and potential applications in hundreds of clinical trials.
A strategic choice to secure a wide range of future treatments by harnessing the potential of mesenchymal stem cells in different medical fields.
A crucial process for preserving one's genetic code, crucial for future medical applications, personalized research and treatments.
Consultation, development, validation, approval, and production, as well as storage in nitrogen vapor, of products in the field of tissue engineering and advanced biological products (Advanced Therapy Medicinal Products, ATMPs) on behalf of third parties.
An innovative service to preserve your immune cells today, with the potential to transform them into induced pluripotent stem cells (iPSC) and access advanced cellular therapies of the future tomorrow.
Reliability
We ensure complete control of all stages of the cryopreservation process, from kit retrieval, through laboratory processing to storage in our tanks. Each step is certified and recorded so that the status of the samples can always be verified.

The only international certification dedicated exclusively to umbilical cord banking

Thanks to this certification, our products have the same quality level as pharmaceutical products.
By choosing SSCB you will opt for a biobank that is committed to exploring the new frontiers of medicine.
International Presence
Our global network extends throughout Europe. Each office and partner reflects our commitment to excellence, working closely together to provide high-quality and secure services and solutions.

Certified Swiss Biotech company a leader in stem cell preservation.
